Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure.[1] Enolization is an example of isomerization, as is tautomerization.[2]
When the activation energy for the isomerization reaction is sufficiently small, both isomers can often be observed and the equilibrium ratio will shift in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, , have been calculated, with good agreement between observed and calculated data.[3]
Examples and applications
[edit]Alkanes
[edit]Skeletal isomerization occurs in the cracking process, used in the petrochemical industry to convert straight chain alkanes to isoparaffins as exemplified in the conversion of normal octane to 2,5-dimethylhexane (an "isoparaffin"):[4]
Fuels containing branched hydrocarbons are favored for internal combustion engines for their higher octane rating.[5] Diesel engines however operate better with straight-chain hydrocarbons.
Alkenes
[edit]Cis vs trans
[edit]Trans-alkenes are about 1 kcal/mol more stable than cis-alkenes. An example of this effect is cis- vs trans-2-butene. The difference is attributed to unfavorable non-bonded interactions in the cis isomer. This effects helps to explain the formation of trans-fats in food processing. In some cases, the isomerization can be reversed using UV-light. The trans isomer of resveratrol converts to the cis isomer in a photochemical reaction.[6]
Terminal vs internal
[edit]Terminal alkenes prefer to isomerize to internal alkenes:
- H2C=CHCH2CH3 → CH3CH=CHCH3
The conversion essentially does not occur in the absence of metal catalysts. This process is employed in the Shell higher olefin process to convert alpha-olefins to internal olefins, which are subjected to olefin metathesis.
Other organic examples
[edit]Isomerism is a major topic in sugar chemistry. Glucose, the most common sugar, exists in four forms.
| Isomers of d-glucose | ||
|---|---|---|
 α-d-glucofuranose α-d-glucofuranose
|
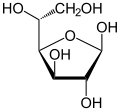 β-d-glucofuranose β-d-glucofuranose
| |
 α-d-glucopyranose α-d-glucopyranose
|
 β-d-glucopyranose β-d-glucopyranose
| |
Aldose-ketose isomerism, also known as Lobry de Bruyn–van Ekenstein transformation, provides an example in saccharide chemistry.[citation needed]
Inorganic and organometallic chemistry
[edit]The compound with the formula (C5H5)2Fe2(CO)4 exists as three isomers in solution. In one isomer the CO ligands are terminal. When a pair of CO are bridging, cis and trans isomers are possible depending on the location of the C5H5 groups.[7]
Another example in organometallic chemistry is the linkage isomerization of decaphenylferrocene, [(η5-C5Ph5)2Fe].[8][9]

See also
[edit]References
[edit]- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "isomerization". doi:10.1351/goldbook.I03295
- ^ Antonov L (2016). Tautomerism: Concepts and Applications in Science and Technology (1st ed.). Weinheim, Germany: Wiley-VCH. ISBN 978-3-527-33995-2.
- ^ How to Compute Isomerization Energies of Organic Molecules with Quantum Chemical Methods Stefan Grimme, Marc Steinmetz, and Martin Korth J. Org. Chem.; 2007; 72(6) pp 2118 - 2126; (Article) doi:10.1021/jo062446p
- ^ Irion, Walther W.; Neuwirth, Otto S. (2000). "Oil Refining". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a18_051. ISBN 3-527-30673-0.
- ^ Karl Griesbaum; Arno Behr; Dieter Biedenkapp; Heinz-Werner Voges; Dorothea Garbe; Christian Paetz; Gerd Collin; Dieter Mayer; Hartmut Höke (2002). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227. ISBN 3-527-30673-0.
- ^ Elyse Bernard, Philip Britz-McKibbin, Nicholas Gernigon (2007). "Resveratrol Photoisomerization: An Integrative Guided-Inquiry Experiment'". Journal of Chemical Education. 84: 1159.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Harris, Daniel C.; Rosenberg, Edward; Roberts, John D. (1974). "Carbon-13 nuclear magnetic resonance spectra and mechanism of bridge–terminal carbonyl exchange in di-µ-carbonyl-bis[carbonyl(η-cyclopentadienyl)iron](Fe–Fe) [{(η-C5H5)Fe(CO)2}2]; cd-di-µ-carbonyl-f-carbonyl-ae-di(η-cyclopentadienyl)-b-(triethyl-phosphite)di-iron(Fe–Fe) [(η-C5H5)2Fe2(CO)3P(OEt)3], and some related complexes" (PDF). Journal of the Chemical Society: Dalton Transactions (22): 2398–2403. doi:10.1039/DT9740002398. ISSN 0300-9246.
- ^ Brown, K. N.; Field, L. D.; Lay, P. A.; Lindall, C. M.; Masters, A. F. (1990). "(η5-Pentaphenylcyclopentadienyl){1-(η6-phenyl)-2,3,4,5-tetraphenylcyclopentadienyl}iron(II), [Fe(η5-C5Ph5){(η6-C6H5)C5Ph4}], a linkage isomer of decaphenylferrocene". J. Chem. Soc., Chem. Commun. (5): 408–410. doi:10.1039/C39900000408.
- ^ Field, L. D.; Hambley, T. W.; Humphrey, P. A.; Lindall, C. M.; Gainsford, G. J.; Masters, A. F.; Stpierre, T. G.; Webb, J. (1995). "Decaphenylferrocene". Aust. J. Chem. 48 (4): 851–860. doi:10.1071/CH9950851.





